The balmer series or balmer lines in atomic physics is one of a set of six named series describing the spectral line emissions of the hydrogen atom the balmer series is calculated using the balmer formula an empirical equation discovered by johann balmer in 1885.
Balmer series solar panels.
Lyman series and balmer series are named after the scientists who found them.
In solar panels light corresponding to the wavelengths in the balmer series is merely reflected by the panel and not absorbed.
The transitions which are responsible for the emission lines of.
Starting with only the balmer series light visible light how could we ensure that the solar panels generate a current that mark can use for his power station.
Since light is not absorbed no current can be produced when the panel is irradiated with light corresponding to the wavelengths in the balmer series.
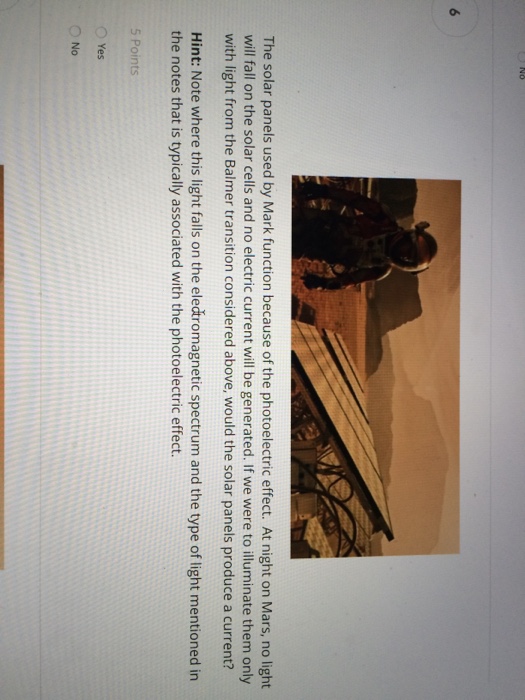
If we were to illuminate them only with light from the balmer transition considered above would the solar panels produce a current.
By gradually increasing the brightness amount of light that we shine on it.
Starting with only the balmer series light visible light how could we ensure that the solar panels generate a current that mark can use for his power station.
Note that the number of solar panels and batteries depends on the system s design and load requirements i e.
The energy levels of hydrogen which are shown in fig.
In astronomy the presence of hydrogen is detected using h alpha line of the balmer series it is also a part.
Multiple batteries and solar panels can be connected in series parallel or series parallel.
By way of comparison with the fairly rough and ready data set obtained above the following is a data set and included regression analysis carried out on wavelengths of balmer series lines up to n 17 obtained from solar eclipses in 1905 and 1925.
All the wavelength of balmer series falls in visible part of electromagnetic spectrum 400nm to 740nm.
The second level which corresponds to n 2 has an energy equal to 13 6 ev 2 2 3 4 ev and so forth.
It may help to look at the electromagnetic spectrum from week 3.
The physicist theodore lyman discovered the lyman series while johann balmer discovered the balmer series.
The key difference between lyman and balmer series is that lyman series forms when an excited electron reaches the n 1 energy level whereas balmer series forms when an excited electron reaches the n 2 energy level.
Balmer series is displayed when electron transition takes place from higher energy states n h 3 4 5 6 7 to n l 2 energy state.
The dominant red fraunhofer line c at wavelength 6563 å is referred to by astronomers as hα of the balmer series.
Choose one 5 points.
1 22 for the lowest level with n 1 the energy is 13 6 ev 1 2 13 6 ev.
1 6 can be obtained by substituting the integer values n 1 2 3 into eq.